This week, Moderna, the biotechnology company centered out of Massachusetts, released results from its second phase of vaccine trials for COVID-19. The company partnered with researchers at the National Institute of Allergy and Infectious Diseases to develop the vaccine.
The results released this week are the first to come from human subjects. The study consisted of 45 subjects, aged 18-55 who were considered to be healthy individuals. They received two vaccinations that were 28 days apart, and after the second dose, all 45 of the participants were shown to produce neutralizing antibodies.
Antibodies are proteins produced by the body’s immune system and target pathogens for destruction. The term “neutralizing” in this context means that in addition to binding to the pathogen, the antibody has the ability to stop the infection, thus “neutralizing” its effects.
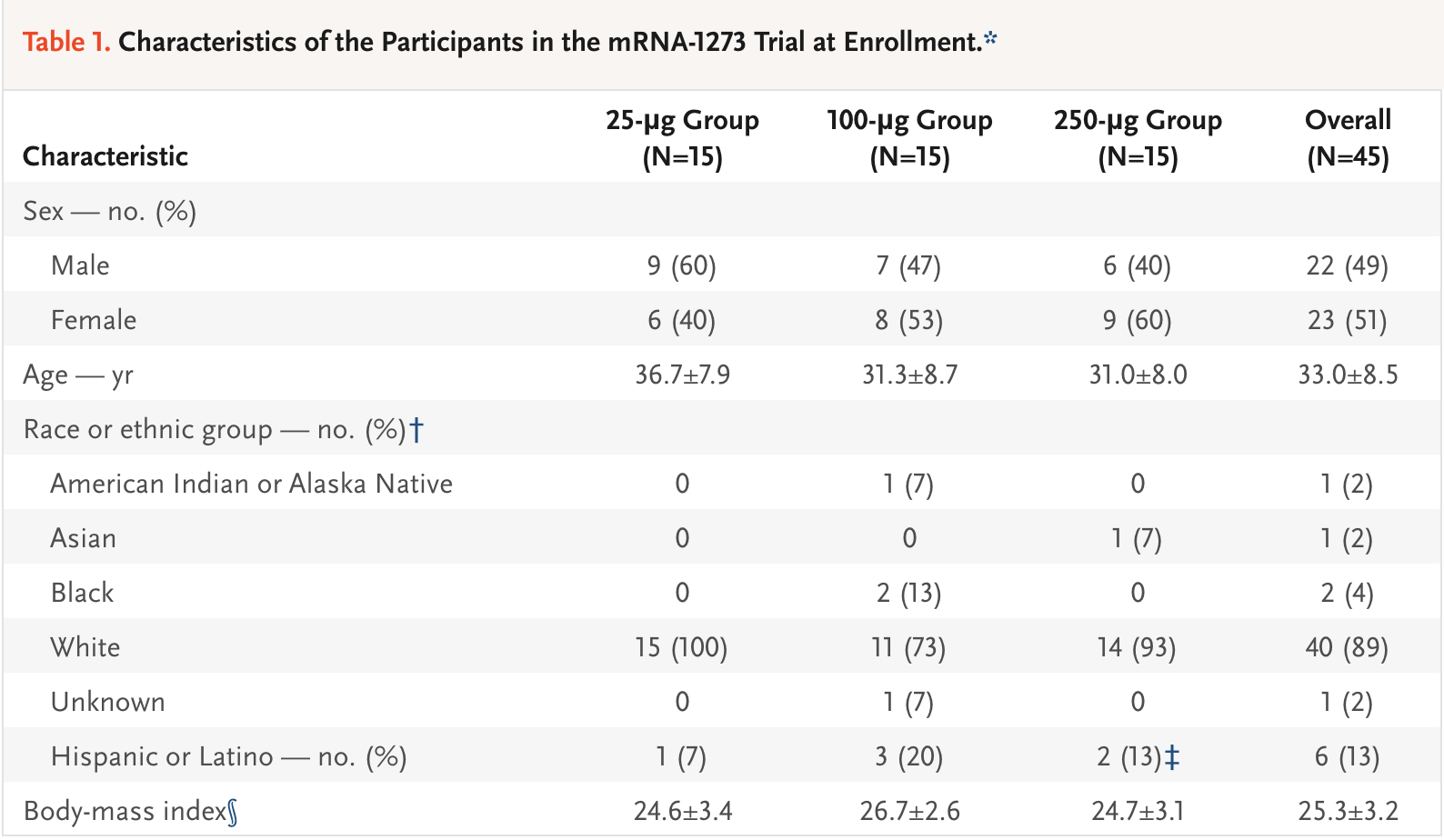
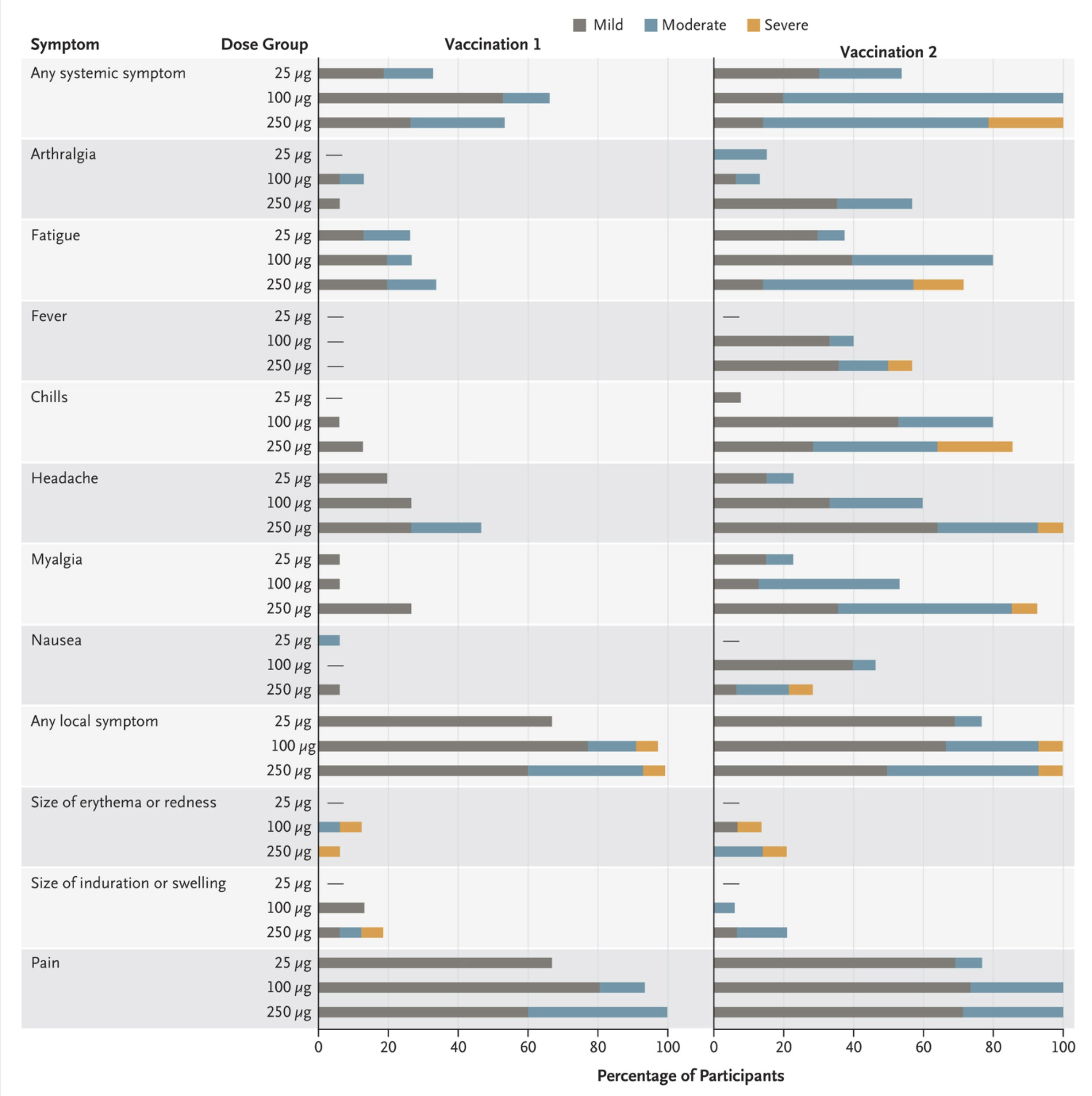
The study included three groups with varying dosage levels of the vaccine. The groups included a 25-microgram (μg) group, a 100-μg group and a 250-μg group. After two vaccinations, researchers measured the signs and symptoms of the subjects and looked for any adverse events. Three participants (21%) in the 250-microgram dose group reported one or more severe adverse events.
RELATED: Is a vaccine the silver lining of the COVID-19 pandemic?
“Solicited adverse events that occurred in more than half the participants included fatigue, chills, headache, myalgia, and pain at the injection site,” the authors wrote. The majority of the subjects in the 25-microgram group reported mild, with some moderate, symptoms in most of the categories, including fatigue, fever, chills or headaches, among many more.
When infected with a virus, a person’s adaptive immune system typically activates two specific types of immunity. The first, cell-mediated immunity, includes T-lymphocytes which help directly kill infected host cells. Humoral immunity is the second division and typically involves the production of antibodies.
When looking at the SARS-CoV-2 virus and COVID-19, the presence of T-cells, in addition to antibodies, in an infected individual is crucial for defense and protection. In the results out of Moderna, “[the] 25-μg and 100-μg doses elicited CD4 T-cell responses [that] … were strongly biased toward expression of Th1 cytokines … with minimal type 2 helper T-cell … expression. CD8 T-cell responses … were detected at low levels after the second vaccination in the 100-μg dose group.”
In essence, these results mean that the vaccine elicited the production of one type of T-cell (CD4) and not the other (CD8). Measuring antibodies is typically easier than T-cells; however, the production of both of these are necessary for a robust immune response against a virus.
Moderna begins its phase 3 trials on July 27 involving 30,000 people. Half of the participants will be a control group who will receive placebos. Russian hackers have been targeting British, Canadian and American organizations researching vaccines using spear-phishing and malware, The New York Times reported.
Amidst growing cases and deaths around the country, a vaccine seems like the glimmer of light at the end of a very dark tunnel. However, as researchers around the world continue to investigate the efficacy and safety of their vaccines, cautious optimism is necessary for staying grounded.
Follow Amit Syal on Twitter