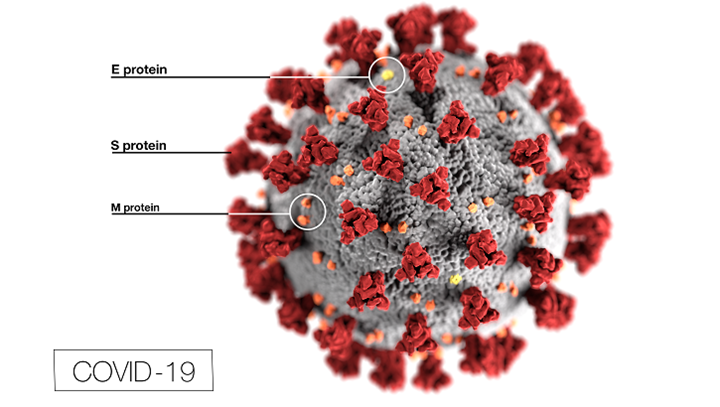
Arguably one of the most important facets of the battle against COVID-19 is the ability for states to increase their testing capabilities. As of May 22, the U.S. has completed over 13 million tests with over 1.7 million coming back positive, according to the Centers for Disease Control.
A new test, in addition to the diagnostic RT-PCR test and the serologic antibody test, has been approved by the Food and Drug Administration recently: the antigen test made by Quidel Corporation. This is the third major type of test to be approved.
RELATED: What are the different tests for COVID-19 and how do they work?
The first test — the diagnostic RT-PCR test — looks for an active infection by testing for the genetic material of the virus. This test typically requires the most equipment but is usually fairly accurate. Typically, patients are swabbed in the nasopharynx region and results typically take a few days to come back.
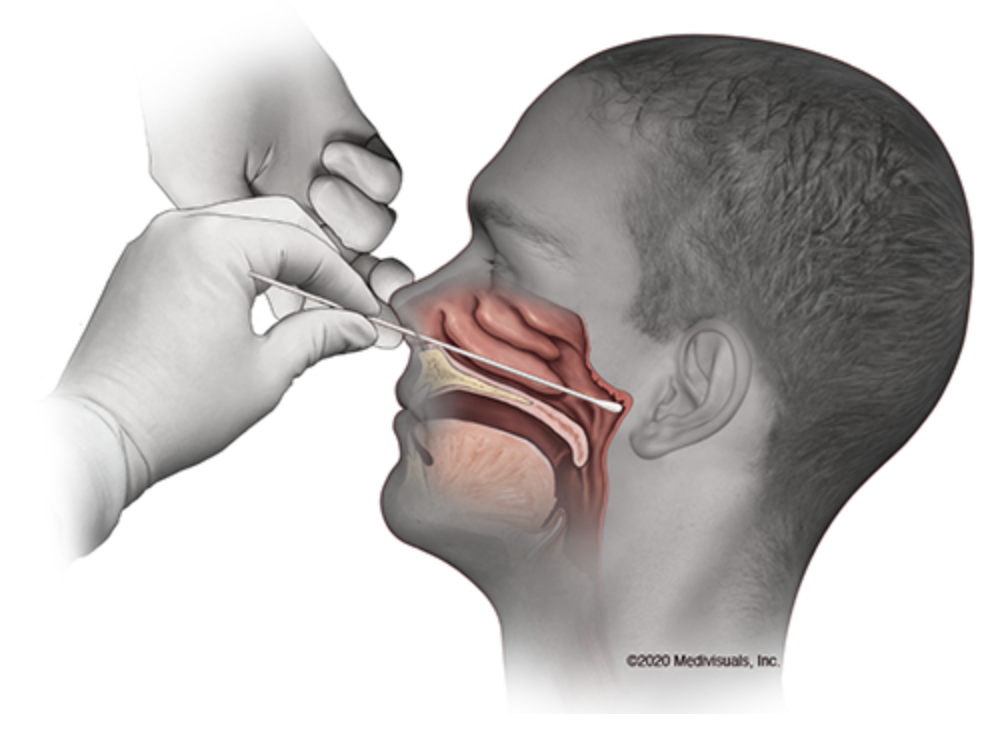
Source: UC Davis Health, MedVisuals
The second test — the serologic antibody test — is extremely useful in terms of a public health and immunity standpoint. This type of test looks for antibodies, as opposed to the RT-PCR, which looks for the genetic material. Antibodies typically confer some level of immunity against a virus, and if this novel coronavirus behaves like pretty much every other virus, then people with antibodies should have immunity.
RELATED: OPINION: I got the antibody test. Here’s how it went.
The third type of test — the antigen test — looks for, as the name implies, antigens, which are foreign substances that elicit the production of antibodies and, in general, an immune response. Antibodies are able to recognize antigens via their epitopes on their surface. The antigen tests diagnose active infections by detecting traces of the virus, in this case, the SARS-CoV-2 virus.
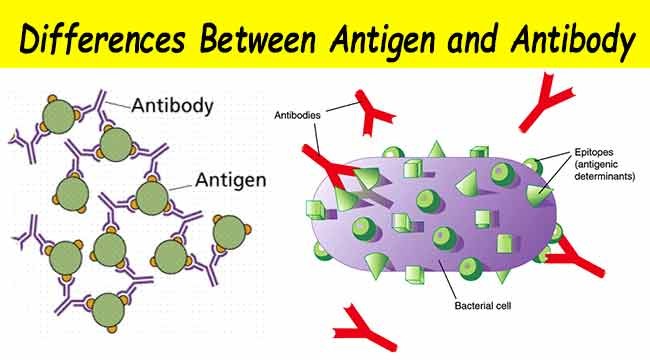
Source: Microbiology Info.
The FDA touts this new form of testing and says that, “[a]ntigen tests are also important in the overall response against COVID-19 as they can generally be produced at a lower cost than PCR tests.”
These tests are typically done via a nasal swab and can produce in 15 minutes. These tests are not as sensitive as RT-PCR tests, meaning that they produce a good number of false negatives, but the results that come out as positive are known to be accurate. In this case, a negative result does not totally rule out infection.
As of May 22, the U.S. currently has over 1.6 confirmed cases of COVID-19 alongside 96,000 deaths. With every single state in the nation partially reopened in some capacity, testing will be absolutely paramount to a safe transition to life before this global pandemic.
Follow Amit Syal on Twitter