On Sunday, Aug. 23, the U.S. Food and Drug Administration announced an emergency use authorization of convalescent plasma therapy to help treat COVID-19.
An emergency use authorization from the FDA does not require the same level of evidence as full FDA approval; however, it increases the availability of a given therapy and “allows FDA to help strengthen the nation’s public health protections.”
This emergency use authorization helps remove some administrative barriers for doctors and other healthcare workers who are using convalescent plasma therapy to treat patients, according to Will Humble, the executive director for the Arizona Public Health Association.
Convalescent plasma is taken from the blood of people who have recovered from COVID-19. The plasma, which contains antibodies against the coronavirus, is given to other patients to help them battle the disease.
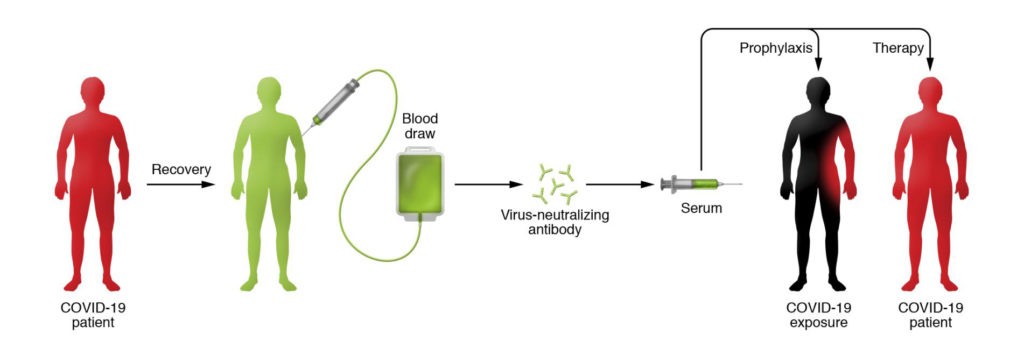
Source: https://www.jci.org/articles/view/138003
According to The New York Times, top health officials such as Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, and Dr. Francis Collins, the director of the National Institutes of Health, thought the current data was not strong enough to warrant an emergency approval.
The convalescent plasma therapy was shown to have a 35% decrease in mortality, according to Dr. Stephen Hahn, commissioner of the FDA, in a press conference on Sunday.
“Although promising, convalescent plasma has not yet been shown to be safe and effective as a treatment for COVID-19. Therefore, it is important to study the safety and efficacy of COVID-19 convalescent plasma in clinical trials,” the FDA said in a press release.
According to Humble, convalescent plasma therapy is most beneficial for patients who are in the early stages of their illness because their own antibodies are yet to form. However, as the illness progresses, the patient will form their own antibodies, rendering the therapy not as effective.
RELATED: Round two: Cracking some common myths about COVID-19
The Mayo Clinic reported its preliminary data, which was published but not yet peer-reviewed, from more than 35,000 patients with COVID-19 who were treated with plasma. The study, which lacked a placebo group, found that quickly administering convalescent plasma had a statistically significant effect on mortality for patients with severe cases of COVID-19.
Those who received transfusions within three days of diagnosis had a seven-day death rate of 8.7%, while patients who got plasma after four or more days had a mortality rate of 11.9%.
“The relationships between mortality and both time to plasma transfusion, and antibody 416 levels provide a signature that is consistent with efficacy for the use of convalescent 417 plasma in the treatment of hospitalized COVID-19 patients,” the study concluded.
However, given the lack of a placebo group in this study, further research and evidence will be needed to bolster the information that was used to declare the emergency use authorization on Sunday.
Follow Amit Syal on Twitter









