Nearly two weeks ago, President Donald Trump, alongside First Lady Melania Trump, tested positive for the coronavirus. In what became his most-liked tweet ever, Trump announced that he would begin the “quarantine and recovery process immediately.”
Last weekend, Trump’s physician Dr. Sean Conley released a memorandum saying Trump “is no longer considered a transmission risk to others.” What treatment did Trump receive in 10 days to go from requiring supplemental oxygen to no longer being infectious, according to his physician?
Regeneron’s monoclonal antibody treatment
The antibody cocktail that Trump received is a combination of two antibodies with an affinity to SARS-CoV-2, the novel strain of coronavirus. These antibodies are able to bind to the surface of the virus, limiting its ability to infect other healthy cells in the body.
The cocktail has yet to undergo a peer-reviewed drug trial despite Trump claiming it to be a “cure.” According to Science, the peer-reviewed academic journal of the American Association for the Advancement of Science, “Researchers plucked the genes for these antibodies from humans who recovered from COVID-19 [and] from mice artificially infected with the virus.”
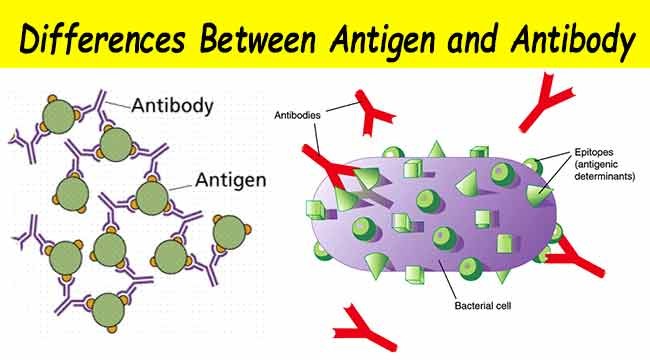
Source: Microbiology Info.
Clinical trials for Regeneron’s monoclonal antibody treatment are ongoing; however, in late September, the company said its treatment reduced the amount of virus in patients taking the drug and accelerated their recovery.
Additionally, according to Regeneron, the treatment also seemed to help patients who hadn’t been hospitalized and had only had mild cases of COVID-19. That raised hopes the monoclonal antibody treatment could work as a general treatment for the disease.
Monoclonal antibodies are more difficult to make than many drugs and often are extremely expensive, which means that demand could outweigh the supply, according to the academic journal Science.
RELATED: New research shows that pain relief from coronavirus may explain large asymptomatic crowd
Dexamethasone
Dexamethasone is a powerful steroid that works by suppressing the body’s natural immune response, thereby helping patients with severe cases of COVID-19. Typically, the use of dexamethasone is reserved for those with serious cases of the disease, giving more insight into the reality of the president’s infection.
“The thought is that by blunting the inflammatory response, we can reduce the severity of severe respiratory failure in patients with COVID-19,” said Dr. Christian Bime, a pulmonary and critical care physician at Banner University Medical Center, earlier in the summer. “We will need to have confirmation from subsequent studies that dexamethasone is indeed a game-changer for COVID-19 respiratory failure.”
According to a study published in the New England Journal of Medicine, in patients hospitalized with COVID-19, the use of dexamethasone resulted in lower 28-day mortality among those who were receiving either invasive mechanical ventilation or oxygen alone at randomization but not among those receiving no respiratory support.
“The president had a high fever and his oxygen saturation was transiently dipping below 94%,” Conley said. A normal blood oxygen saturation level is 95% or higher, meaning that Trump was in need of oxygen supplementation to better cope with his illness.
“This medication is used in [COVID-19] patients that have low oxygen saturation on room air. It tamps down inflammation and has been shown to improve clinical outcomes in patients that have lowered blood oxygen levels,” said Will Humble, the executive director of the Arizona Public Health Association.
As a side effect, steroids, like dexamethasone, may also affect mood, causing a feeling of euphoria or general happiness, as a result of excess plasma dopamine being released. Steroids can also disrupt sleep, leading to insomnia, irritability or depression.
RELATED: New study finds less than 10% of Americans have antibodies against novel coronavirus
Remdesivir
Remdesivir is an antiviral drug that is commonly taken alongside dexamethasone to aid in the inhibition of viral RNA production. Remdesivir is an experimental drug that is used in hospitalized patients and has been granted an emergency use authorization by the U.S. Food and Drug Administration.
This authorization enables doctors to prescribe Remdesivir to patients without enrolling them in a clinical trial or getting compassionate use approval, according to Humble.
A study published in the New England Journal of Medicine found that patients receiving Remdesivir recovered in 11 days, compared to 15 days for those who received a placebo. However, that study found the medication to be most effective for patients who were hospitalized with COVID-19 and required supplemental oxygen.
Follow Amit Syal on Twitter