The United States is averaging upwards of 150,000 new coronavirus cases per day this week as major cities around the country, including New York, Philadelphia and Chicago, have implemented new restrictions. Yesterday, the country passed the grim milestone of 250,000 deaths from the coronavirus as winter approaches.
On Tuesday, the U.S. Food and Drug Administration authorized the first at-home coronavirus test as another measure to curb the spread of the virus and mitigate future outbreaks. The Lucira testing kit is a molecular single-use test that is intended to detect the novel coronavirus, SARS-CoV-2.
RELATED: Moderna releases interim coronavirus vaccine data, over 94% effective
“The FDA continues to demonstrate its unprecedented speed in response to the pandemic,” said FDA Commissioner, Dr. Stephen Hahn, in a statement. “While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home … . Today’s action underscores the FDA’s ongoing commitment to expand access to COVID-19 testing.”
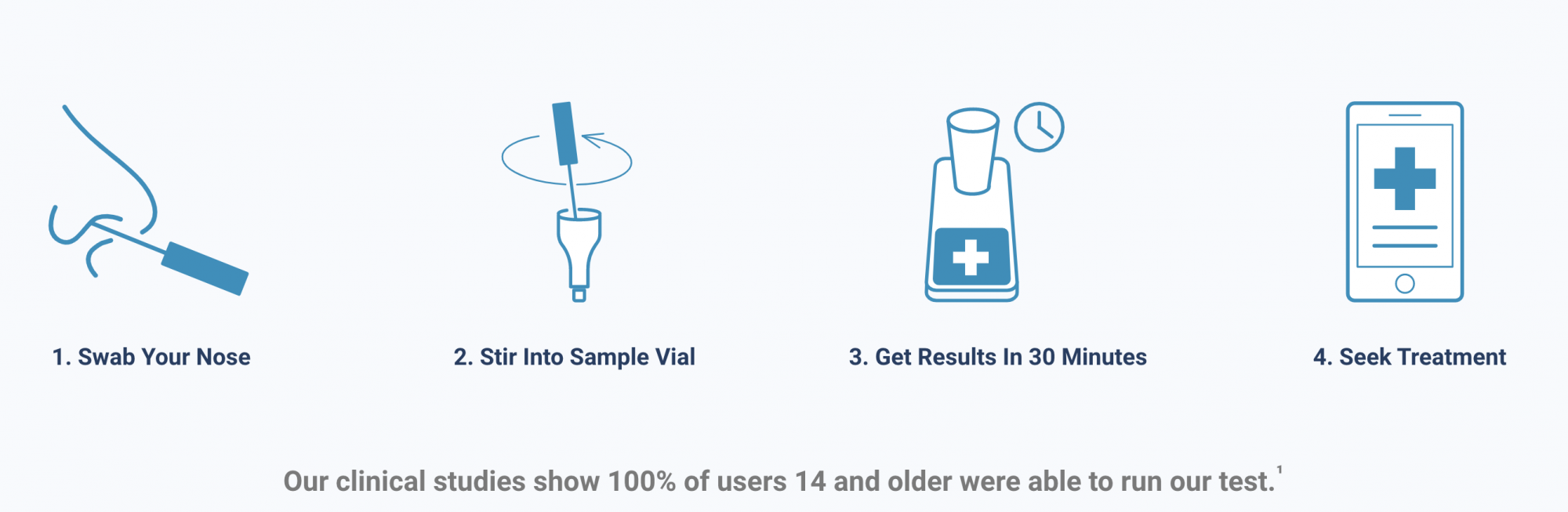
Source: Lucira Health
According to the FDA, the test works by swirling the self-collected sample swab in a vial that is then placed in the test unit. In 30 minutes or less, the results can be read directly from the test unit’s light-up display that shows whether a person is positive or negative for the coronavirus.
The new at-home test relies on similar principles by using a method called a loop-mediated amplification reaction. Like a polymerase chain reaction, LAMP repeatedly copies genetic material until it reaches detectable levels, making it possible to identify the virus even when it is present at only very low levels in the respiratory tract. While faster and less cumbersome than PCR, LAMP is generally thought to be less accurate.
Lucira Health, the test manufacturer, has also developed box labeling, quick reference instructions and health care provider instructions to assist with reporting, according to the press release. With a relatively simple nasal swab, the coronavirus test can return results in about 30 minutes and is projected by the company to cost $50 or less, according to the product’s website.
“Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them,” said Dr. Jeff Shuren, director of FDA’s Center for Devices and Radiological Health. “We look forward to proactively working with test developers to support the availability of more at-home test options.”
Follow Amit Syal on Twitter